The ‘Medical Implant Project,’ but more widely known as the Medical Device Information System is a nation-wide project for public and private healthcare sectors are tackling to record and track implant and some surgical patient data. Jo Doherty, the Project Lead and Kristin Lee, the Project Manager have been working alongside the Trust and external sources, such as NHS Digital and NHSx to simplify and automate the data process to ensure patient safety.
The Challenge
Background
NHS Digital are establishing a Medical Device Information System (MDIS), which will operate in accordance with regulations that will set out how medical device information from NHS and private healthcare providers across the UK is collected, analysed and used.
MDIS will build on the design and base infrastructure of the Surgical Devices and Implants Information System and will help trace high-risk implantable medical devices over the next 3-5 years. This project was assigned in July 2020 and Trusts were required to start submitting data in July 2021.
In time, the system will enable interoperable message exchange and alerting across the health and care system. This will ensure a consistent UK wide approach to the monitoring of medical device safety. It will allow recalls and alerts where issues are identified and will provide an improved flow of appropriate data to clinical registries and regulators.
Why change the current process?
The current system of how medical devices are purchased, used and monitored, whilst still being focused on safety, does not provide for the systematic linking of devices with the patients they are used on.
There is a need for more information to be collected and shared to identify risks of specific devices early. This will allow patients and clinicians to discuss any necessary interventions, prevent harm and keep patients safe.
Policy
Regulations made under the MMD bill will facilitate the tracking of higher-risk implanted medical devices across the UK so that their performance and outcomes can be monitored. It will also enable issues to be identified earlier so that clinicians can rapidly intervene, if needed, and mitigate risks faced by patients.
The bill:
- Provides a power to make regulations setting out how an information system will operate and how information will be collected from healthcare providers and other relevant private healthcare providers across the UK
- Provides for full public consultation prior to the regulations being laid, including consultation with the devolved administrations
- Through regulations, will allow medical devices to be linked by their unique identifier to a patient’s individual record, and patient and procedure data on devices to be collected and used to improve patient safety
Our biggest issue with this project was that a majority of patient data was manual as in hand written and some information was on a database, but with not way to clearly filter the information. For a year, to collate data it was manually collecting the data from various sources, such as APAS, patient folders, stickers, etc. and piece it together like a puzzle, which was time consuming until the Trust implemented Cerner Trust wide in May 2022.
Our Solution
In relation to the outlined challenge above, the project manager, Kristin Lee, had the Trust Informatics Team create a reports system through the new Cerner system, so the data for the necessary departments could be exported any time and get submitted to MDIS.
Results
We are now able to submit data weekly, and easily add more departments as and when necessary.
“The activity by Kristin Lee on the device data collation and project submission development has been instrumental to the actualization of Royal Surrey’s submission to the Pelvic Floor Registry (MDIS). This in turn will ensure patient safety in the use of medical devices and implants, and the fulfilment of the requirements raised by the medical Devices Safety Review: First Do No Harm (Cumberlege Report).
Particularly, Kristin’s research on systems interoperability and information governance expedited the development and authorization of submission that allowed realization of the benefits of the data usage earlier than otherwise possible.”
–Sean Ryan, NHS Digital Data Liaison
Related media
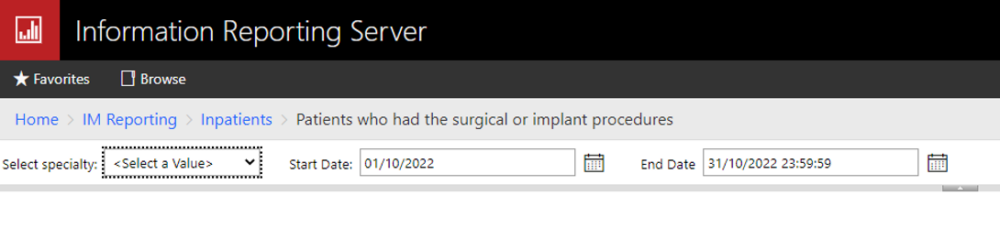
Our reports system menu, generated by the Informatics Team to export the necessary Cerner data for MDIS submission:
The image below is the Surgical Devices and Implant Directions 2020 given to the Chief Executive at NHS Digital to mandate this for Trusts across the UK

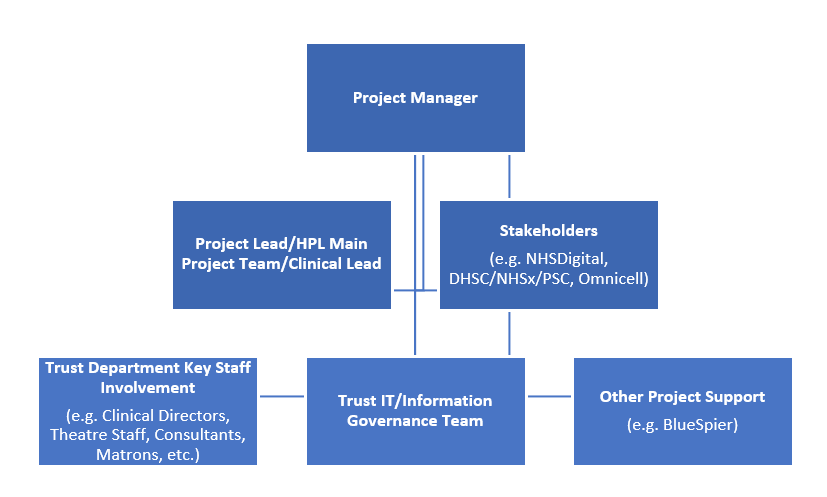
This was the team and communication structure:
Visual Reinforcement Audiology room (VRA) for Kingston Hospital
Discover how we designed and installed a bespoke VRA room for Kingston Hospital — enabling quick and...
Wound Care Centralised Ordering Project within the Surrey Heartlands ICP’s
Our successful delivery of centralised wound care for the Surrey Highlands ICP transformed a system characterised by...